Digital Health
FDA says yes to MedCognetics breast cancer screening AI
Digital health company MedCognetics has picked up an FDA approval for QmTRIAGE, its artificial intelligence-powered software for detecting
The post FDA…
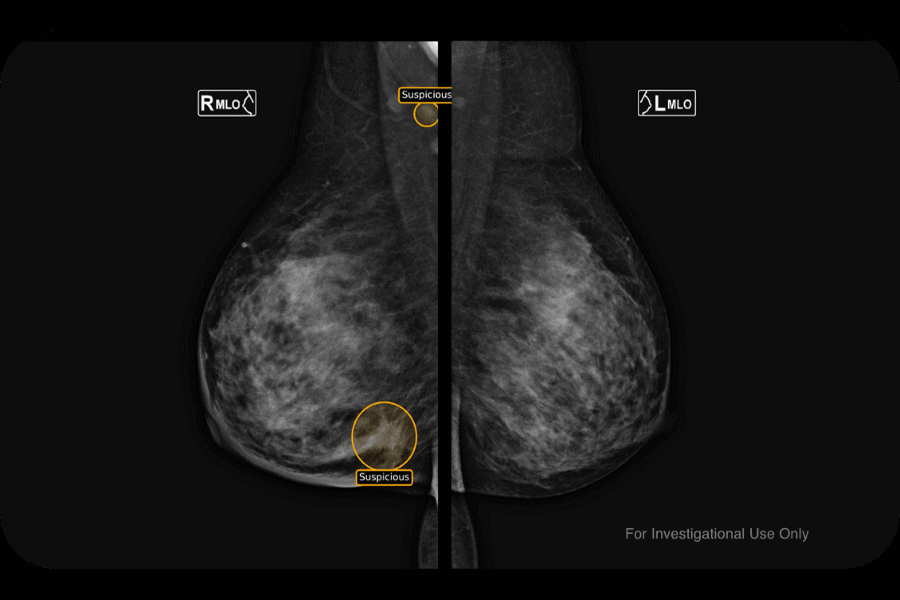

Digital health company MedCognetics has picked up an FDA approval for QmTRIAGE, its artificial intelligence-powered software for detecting breast cancer in medical images.
The company said QmTRIAGE can help alleviate a chronic shortage in radiology services around the world.
It cites data estimating there is ten-fold higher demand for radiology services than available capacity, with around half of practitioners “experiencing depression or burnout” due to “unmanageable caseloads.”
Dallas-based MedCognetics says the cloud-based AI software as a service (SaaS) has been trained on a diverse patient dataset harvested from around the world to reduce data bias, and can detect the earliest manifestations of cancer in all ethnicities.
It slots into the standard radiology workflow, so the radiologist does not have to engage in any additional tasks during the process, with the AI’s findings layered on top of mammography images.
Breast cancer is a leading cause of death among women worldwide and many countries have introduced mammography screening programmes to detect and treat it early. However, examining mammograms for early signs of cancer is high volume, repetitive work for radiologists, and some cancers are missed.
MedCognetics claims its digital health software reduces the time and cost of imaging, and can improve outcomes across diverse patient populations.
It trained its AI on deidentified breast imaging data gathered by the University of Texas and UT Southwestern Medical Centre (UTSW) in Dallas, which hold equity in the company and licensed some intellectual property to it.
“The American Cancer Society has stated that, in 2022, approximately 287,850 new cases of invasive breast cancer will be diagnosed in women,” said Debasish Nag, the company’s chief executive. “Our software’s high detection accuracy enables reduced time for review by radiologists, another key component to improved outcomes.”
He described the FDA 510(k) approval of QmTRIAGE as “an important first step for us as we work toward expanding to other realms of cancer.”
While AI has emerged as an additional tool that can assist radiologists in their workload, a study published in the British Medical Journal last year concluded that the technology is not yet ready to replace a trained practitioner.
The analysis of a dozen studies of commercially-available AI-powered image recognition software for breast cancer screening found there was no basis for claims made by some developers that using AI for breast screening can be superior to review experienced radiologists.
The authors acknowledged, however, that AI algorithms are constantly improving and might change in the future – although, randomised controlled trials should be undertaken to evaluate how they perform in combination with radiologists.
The post FDA says yes to MedCognetics breast cancer screening AI appeared first on .
digital health
artificial intelligence
ai
software
medical
fda

Keep it Short
By KIM BELLARD OK, I admit it: I’m on Facebook. I still use Twitter – whoops, I mean X. I have an Instagram account but don’t think I’ve ever posted….
Asian Fund for Cancer Research announces Degron Therapeutics as the 2023 BRACE Award Venture Competition Winner
The Asian Fund for Cancer Research (AFCR) is pleased to announce that Degron Therapeutics was selected as the winner of the 2023 BRACE Award Venture Competition….
Seattle startup Olamedi building platform to automate health clinic communications
A new Seattle startup led by co-founders with experience in health tech is aiming to automate communication processes for healthcare clinics with its software…













