Medtech
As money pours into digital therapeutics, insurance coverage crawls
Talk therapy didn’t help Lily with attention deficit hyperactivity disorder, or ADHD. But a video game did.
As the 10-year-old zooms through icy waters…
Talk therapy didn’t help Lily with attention deficit hyperactivity disorder, or ADHD. But a video game did.
As the 10-year-old zooms through icy waters and targets flying creatures on the snow-capped planet Frigidus, she builds attention skills, thanks to Akili Interactive Labs’ video game EndeavorRx. She’s now less anxious and scattered, allowing her to stay on a low dose of ADHD medication, according to her mom Violet Vu.
In a clinical trial conducted by Duke Clinical Research Institute with 206 kids, 68% of parents reported improvements in ADHD-related symptoms after two months of use, according to Akili. But Violet Vu said her insurance doesn’t cover EndeavorRx, leaving the family to pay $99 a month out of pocket.
“That was a tough thing,” she said. “There were some months we were like, ‘Gosh, is this something that we should be paying towards?’”
In 2020, EndeavorRx became the first video game to win FDA authorization as a mental health treatment. Despite regulatory traction, insurers have been slow to cover EndeavorRx and other digital therapeutics, and many doctors remain unaware of these therapies.

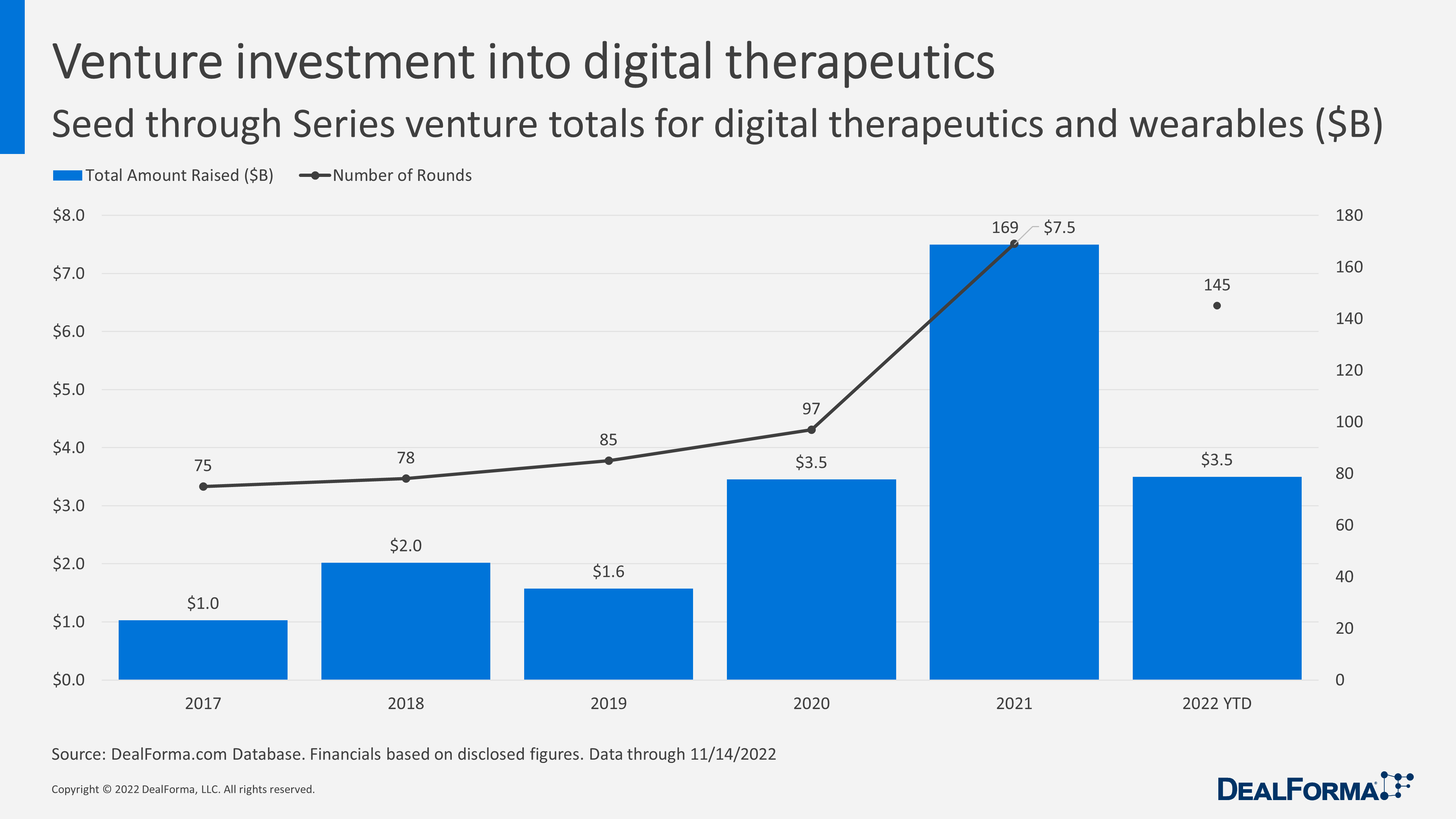
The digital therapeutics space saw a record $7.5 billion in investment last year after 169 funding rounds, according to Chris Dokomajilar at DealForma. But for the industry to reach the mainstream, companies are trying to win over private insurers while advocating for legislation that would compel government coverage.
Vu said the family’s prescriptions are managed by Express Scripts, a subsidiary of Evernorth. While Evernorth said it believes in the “potential of digital therapeutics to create value,” it added that coverage decisions ultimately come down to a patient’s health plan or sponsor. Express Scripts and Evernorth recently published a digital health formulary to help guide those coverage decisions, but EndeavorRx is not included on the list.
 Eddie Martucci
Eddie Martucci“No one envisioned 10 years ago these products. But now they’re here, and they’re being prescribed to patients all over the country, so we do need categories so that we can have things like government payment thresholds,” Akili CEO Eddie Martucci said.
Anthem — one of the country’s largest insurers — has determined that EndeavorRx and a slate of other digital therapeutics aren’t “medically necessary.”
“We review all policy documents at least once a year, but often more frequently if we become aware of substantive new data,” an Anthem spokesperson said in a statement. Anthem, which recently rebranded as Elevance Health, declined to provide more information on its policy decision.
Why go digital?
Digital therapeutics encompass much more than ADHD, and aim to offer an alternative to traditional medicine.
Many with post-traumatic stress disorder, for example, are given antidepressants that may carry onerous side effects ranging from dry mouth to insomnia. Other drugs such as benzodiazepines — which the Departments of Defense and Veterans Affairs have recommended against — are often abused.
In contrast, Freespira’s medical device helps patients regulate their breathing using a breathing tube connected to a tablet. Patients are encouraged to participate in two 17-minute sessions a day, and they can also access training and weekly check-ins with a coach.
 Joe Perekupka
Joe Perekupka“What we’re doing which is uniquely different is we’re [teaching] patients a lifelong skill that will allow them to stabilize their breathing, so that when they hit those highly anxious periods that would normally trigger a panic attack, they won’t actually have the panic attack,” Freespira CEO Joe Perekupka said.
While the company has celebrated some insurance wins, it struggled to get coverage upon launching a few years ago. Other digital therapeutic makers have reported the same.
 Jayne Hornung
Jayne HornungLast year, the research and consulting group Managed Markets Insight & Technology (MMIT) surveyed 16 payers and found that just a quarter were willing to cover prescription digital therapeutics. About 76% rated them as a low to moderate priority, part of the reason being that physicians aren’t always aware of digital therapeutics, according to Jayne Hornung, MMIT’s chief clinical officer, pharmacy.
“They didn’t have the requests from physicians and the requests from employers to make it a priority,” Hornung said. “A lot of physicians aren’t even familiar with digital therapeutics, how they work, how they can benefit a patient, the studies behind them.”
Matt Fickie, senior medical director at the insurance company Highmark, said insurance coverage for digital therapeutics has moved at a glacial pace. But that’s starting to change.
“Silicon Valley works a lot faster than insurance companies,” he said. “It’s really a clash of cultures.”
Grasping for coverage
 Corey McCann
Corey McCannPear Therapeutics, which develops mobile applications for conditions such as substance abuse disorder and insomnia, forecasted it would reach up to 60,000 subscribers this year. While “demand is quite strong,” according to McCann, he now predicts Pear will achieve roughly two-thirds that subscriber amount, in part because payers are moving “more slowly than we would prefer.”
Using Pear’s software for substance use disorder, patients can report cravings, triggers and drug screen results. The app includes more than 60 lessons, such as “Coping with Thoughts About Using,” and rewards patients for staying on track with gift cards or virtual rewards.
However, with the advent of new insurance codes that allow for more direct billing — and a rise in telehealth — insurers are slowly warming up. Highmark in April began covering FDA-authorized digital therapeutics for certain patients with a prescription, including Pear’s products. However, Fickie believes there’s still a ways to go in data collection across the field.
“If I were able to talk to developers, it would be to work on their evidence,” he said, adding that he wants to see more long-term data showing patients correctly use products and improve based on commonly used medical scales.
Real-time data monitoring has earned some skepticism, as patients wonder just how much information researchers — and payers — will see.
“Both consumer and clinical grade apps and technologies collect a lot of data, but it is unclear whether consumers are given sufficient information to make an informed choice regarding the sharing of their data,” Aloha McBride, an EY global health leader, wrote in a MedTech Dive opinion piece last year.
 Andy Molnar
Andy MolnarAndy Molnar, CEO of the nonprofit Digital Therapeutics Alliance, acknowledged data privacy concerns, but said companies must comply with HIPAA. And, he added, digital therapeutics suck up data that insurers already collect in more traditional ways.
“They know every time you went to the hospital, every time you go to the doctor and what codes were billed when you were there. They know if you’re taking your medication, basically, based on refills,” he said. “All that information is out there.”
Justin Birckbichler experienced the power of real-time data firsthand. When he was in remission from testicular cancer and needed mental health help, he turned to an online program, eTC Express. It helps men in remission from testicular cancer manage their anxiety and distress.
After a particularly tough week, Birckbichler rated his mood low in the online app, and soon after, eTC Express’ lead researcher reached out to help him. Other apps, because they aren’t as data-centric or monitored as consistently, haven’t offered the same level of service.
“There were some days where I put in that I was stressed, and I never heard from the CEO… or anything like that,” Birckbichler said of the other apps.
Taking the fight to Capitol Hill
Another lever for increasing coverage: legislation. The Access to Prescription Digital Therapeutics Act, introduced in March and currently in the Senate Finance Committee, would create a coverage definition for digital therapeutics and create a pathway for Medicare and Medicaid reimbursement.
“Financial models are going to be important, both in terms of coverage by Medicare or private insurance,” said Stanley Shaw, a Harvard professor who teaches a class on digital health. “There’ll be a protracted need for education for patients; some of these are for kids. So you’ve got to educate parents or caregivers and also doctors.”
As Lily nears her 11th birthday, she continues to play EndeavorRx as part of her daily treatment routine. During short periods when Lily stopped playing, she subtly backslid, her mom said.
Because EndeavorRx still isn’t covered by insurance, the family continues to pay out-of-pocket despite the financial burden.
“It’s not a silver bullet per sé. Nothing is,” Violet Vu said. “But it’s a really good item to add to that repertoire. So she’s doing really good, she’s finally kind of thriving.”
therapeutics
medical
medicine
health
hospital
prescription
device
digital health
telehealth
apps
software
mobile
therapy
authorization
fda
research
clinical research
markets
rated

ETF Talk: AI is ‘Big Generator’
Second nature comes alive Even if you close your eyes We exist through this strange device — Yes, “Big Generator” Artificial intelligence (AI) has…
Apple gets an appeals court win for its Apple Watch
Apple has at least a couple more weeks before it has to worry about another sales ban.
Federal court blocks ban on Apple Watches after Apple appeal
A federal appeals court has temporarily blocked a sweeping import ban on Apple’s latest smartwatches while the patent dispute winds its way through…














