Life Sciences
ASX Health Stocks: Neuren surges 20pc on ‘historic’ FDA approval, HITIQ snaps up Monash Uni as customer
Neuren Pharmaceuticals says its North American partner Acadia Phama has received a “historic” US FDA approval of DAYBUE (trofinetide). … Read More
The…
- Neuren Pharma gets a ‘historic’ FDA approval for DAYBUE to treat Rett syndrome
- Genetic Technologies published another research paper
- HITIQ sells 200 Nexus Head Impact Sensors to Monash University
Neuren Pharmaceuticals (ASX:NEU) surged 20% this morning after announcing that its North American partner Acadia Pharma has received a “historic” US FDA approval of DAYBUE (trofinetide) for the treatment of Rett syndrome.
Acadia expects DAYBUE (a brand name for trofinetide) to be available by the end of April to treat adult and pediatric patients two years of age and older suffering from the disease.
DAYBUE is the first and only approved treatment for Rett syndrome ever in the world.
Now read: Boost for ASX healthcare sector as Neuren gets US FDA approval for drug to treats Rett’s syndrome
“Many people have shown great determination over the long journey to reach this historic outcome,” said Neuren CEO, Jon Pilcher.
“The greatest has been shown by the Rett syndrome community, and I am delighted for them.”
“For Neuren, this is a transforming milestone that places us in a position to make the most of the opportunities ahead of us, as we work with the communities to make a difference in four other neurodevelopmental disorders,” Pilcher added.
Neuren set to receive millions in payments
In October 2022, Neuren received from Acadia a milestone payment of US$10 million following the acceptance of the NDA for review by the FDA.
The next milestone payment to Neuren is US$40 million, payable following the first commercial sale of trofinetide in the United States, which is anticipated at the end of April 2023.
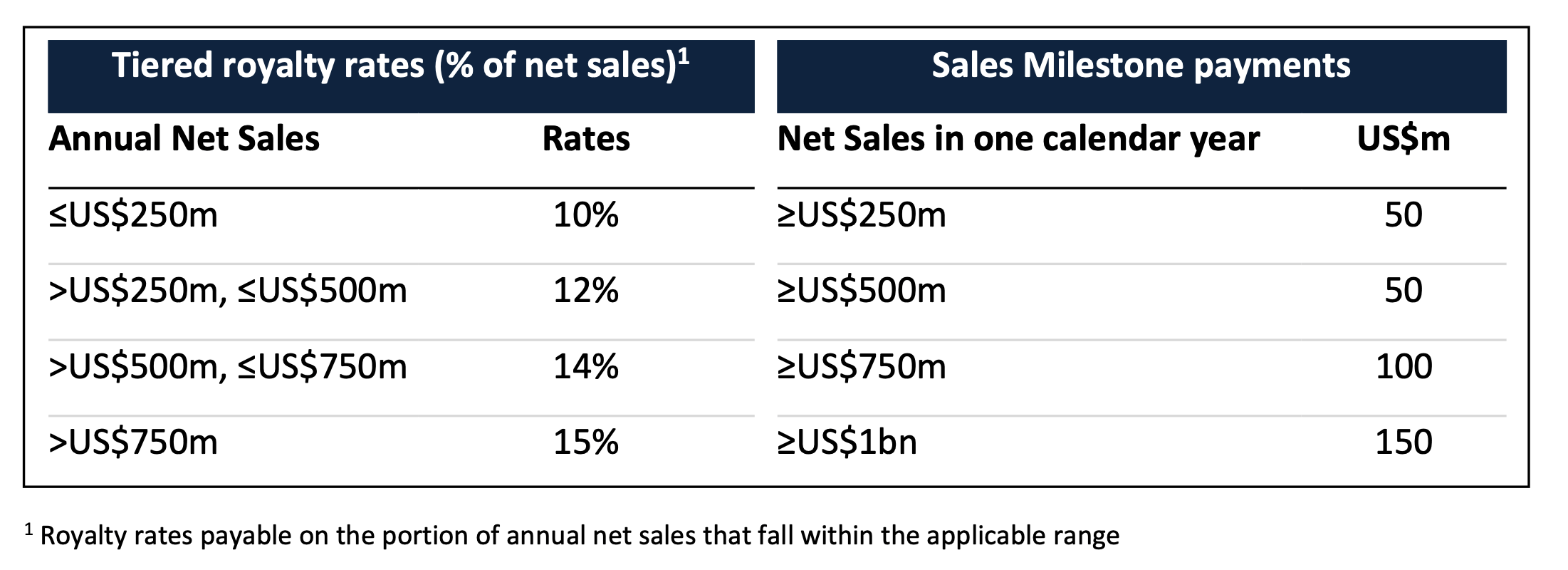
Neuren is also eligible to receive ongoing royalties on net sales of trofinetide in North America, plus milestone payments of up to US$350 million on achievement of a series of four thresholds of total annual net sales.
The company is also set to earn one third of the market value of the Rare Pediatric Disease Priority Review Voucher that was awarded to Acadia by the FDA upon approval of the NDA, estimated at US$33 million.
The royalty rates and sales milestone payments are related to the total amount of annual net sales of trofinetide in all indications, as set out in the following table:

Neuren retains all rights to trofinetide for all countries outside North America, and has a fully paid-up, irrevocable licence for use in those countries to all data generated by Acadia.
Neuren intends to pursue registration and commercialisation of trofinetide through partners, and is currently advancing discussions with a number of third parties.
Currently Neuren does not have the necessary approvals or available drug supply to enable any compassionate use or named patient programs.
Neuren share price today:
Other notable ASX announcements today
HITIQ has secured sale of 200 Nexus Head Impact Sensors to Monash University to investigate the effects of head impacts in Australian Football.
Monash is aiming to pioneer an Artificial Intelligence (AI) model that will translate HITIQ’s head impact data into a quantified risk of concussion.
The University is one of Australia’s leading universities for neuroscience, and is globally ranked 44 in the Times Higher Education World University Rankings (2023).
Genetic Technologies (ASX:GTG)
GTG announced the publication of another research paper supporting the utilisation of its geneType Breast Cancer Risk Assessment Test in the peer- reviewed journal Cancer Prevention Research.
This is the second published peer-reviewed paper in the last month supporting the use of geneType.
The geneType Breast Cancer Risk Assessment Test has demonstrated improved performance compared to a gold- standard model (BCRAT) over a 5-year period.
Race says it has appointed the Contract Research Organisations (CRO) Resolutum Global, Beyond Drug Development, and NSW Regional Biospecimen & Research Services, to support the observational stage of a Phase 1/2b clinical trial of Zantrene (bisantrene).
The trial will study breast cancer patients treated with doxorubicin and cyclophosphamide, and who have two or more cardiovascular risk factors.
The clinical trial institutional governance submission is expected soon, and will enable enrolment of the first study patient.
Share prices today:
The post ASX Health Stocks: Neuren surges 20pc on ‘historic’ FDA approval, HITIQ snaps up Monash Uni as customer appeared first on Stockhead.
contract research
cro
pharmaceuticals
pharma
healthcare
fda
research

Wittiest stocks:: Avalo Therapeutics Inc (NASDAQ:AVTX 0.00%), Nokia Corp ADR (NYSE:NOK 0.90%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Spellbinding stocks: LumiraDx Limited (NASDAQ:LMDX 4.62%), Transocean Ltd (NYSE:RIG -2.67%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Asian Fund for Cancer Research announces Degron Therapeutics as the 2023 BRACE Award Venture Competition Winner
The Asian Fund for Cancer Research (AFCR) is pleased to announce that Degron Therapeutics was selected as the winner of the 2023 BRACE Award Venture Competition….














