Life Sciences
Cancer Cell-Based Vaccine Kills Tumors and Induces Long-Term Immunity
Researchers at Brigham and Women’s Hospital devised a new way to turn cancer cells into potent anticancer agents. The cell therapy approach is harnesses…
Researchers headed by a team at Brigham and Women’s Hospital have devised a new way to turn cancer cells into potent anticancer agents. Scientists in the lab of Khalid Shah, PhD, developed a cell therapy approach to eliminate established tumors and induce long-term immunity, training the immune system so that it can prevent cancer from recurring. The team tested their dual-action, cancer-killing vaccine in an advanced mouse model of the deadly brain cancer glioblastoma (GBM), with promising results.
“Our team has pursued a simple idea: to take cancer cells and transform them into cancer killers and vaccines,” said Shah, director of the Center for Stem Cell and Translational Immunotherapy (CSTI) and the vice chair of research in the department of neurosurgery at the Brigham and faculty at Harvard Medical School and Harvard Stem Cell Institute (HSCI). “Using gene engineering, we are repurposing cancer cells to develop a therapeutic that kills tumor cells and stimulates the immune system to both destroy primary tumors and prevent cancer.” Shah is corresponding author of the team’s published paper in Science Translational Medicine, which is titled, “Bifunctional cancer cell-based vaccine concomitantly drives direct tumor killing and antitumor immunity,” and in which they concluded, “Here, we developed a bifunctional therapeutic strategy by transforming living tumor cells into a potent agent that concomitantly drives direct tumor killing and antitumor immunity.”
Cancer vaccines are an active area of research for many labs, and it has been shown that administering inactivated tumor cells can induce a potent antitumor immune response. However, the efficacy of this approach is limited by the inability to kill tumor cells before inducing the immune responses, the authors explained. “Phase I to III clinical trials have tested the therapeutic efficacy of inactivated tumor cells for various types of cancer … However, this therapeutic approach showed limited or no clinical benefit, which could be attributed to the lack of direct cytotoxic effect on tumor cells and the inability to trigger a strong antitumor immune response.”
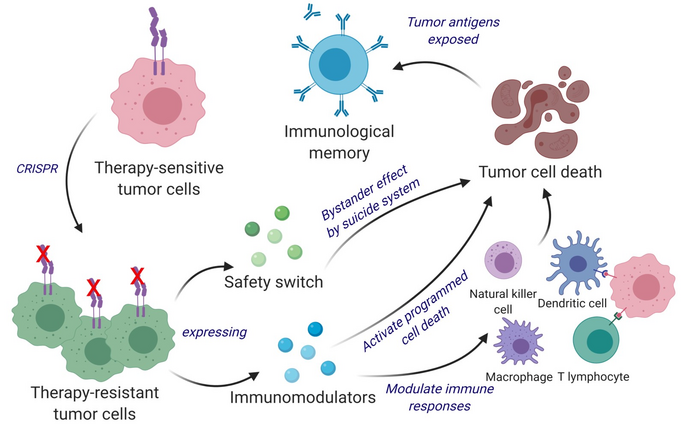
Shah and colleagues have instead devised a different strategy that repurposes living tumor cells, which possess an unusual feature. Like homing pigeons returning to roost, living tumor cells will travel long distances across the brain to return to the site of their fellow tumor cells. Taking advantage of this unique property, Shah’s team used CRISPR-Cas9 gene editing technology to engineer living tumor cells and repurpose them to release a tumor cell killing agent. The engineered tumor cells were in addition designed to express factors that would make them easy for the immune system to spot, tag, and remember, priming the immune system for a long-term antitumor response.
“We first used CRISPR-Cas9 to knock out the IFNβ-specific receptor (IFNAR1) in inherently IFNβ-sensitive syngeneic tumor cells and subsequently engineered them to constitutively produce IFNβ for tumor cell targeting and simultaneous immunomodulation,” they explained. “These therapeutic cells are further designed to coexpress granulocyte macrophage colony-stimulating factor (GM-CSF) that facilitates the differentiation, proliferation, and recruitment of dendritic cells (DCs).”
The team tested their repurposed CRISPR-enhanced and reverse-engineered therapeutic tumor cells (ThTC) in different mice strains, including one with bone marrow, liver, and thymus cells derived from humans, mimicking the human immune microenvironment. The researchers also built a two-layered safety switch into the cancer cell, which, when activated, eradicates ThTCs if needed.

Their experiments demonstrated that the dual-action cell therapy was safe, applicable, and efficacious in the models, suggesting a roadmap toward therapy. While further development and testing will be needed, Shah’s team specifically chose this model and used human cells to smooth the path of translating their findings for patient settings.
“These engineered therapeutic tumor cells (ThTCs) eliminated established glioblastoma tumors in mice by inducing caspase-mediated cancer cell apoptosis, down-regulating cancer-associated fibroblast-expressed platelet-derived growth factor receptor β, and activating antitumor immune cell trafficking and antigen-specific T cell activation signaling,” the investigators stated. “This mechanism-based efficacy of ThTCs translated into a survival benefit and long-term immunity in primary, recurrent, and metastatic cancer models in immunocompetent and humanized mice.”
Shah added, “Throughout all of the work that we do in the Center, even when it is highly technical, we never lose sight of the patient. Our goal is to take an innovative but translatable approach so that we can develop a therapeutic, cancer-killing vaccine that ultimately will have a lasting impact in medicine.”
Shah and colleagues noted that this therapeutic strategy is applicable to a wider range of solid tumors and that further investigation of potential applications is warranted. “Here, we developed a bifunctional therapeutic strategy by transforming living tumor cells into a potent agent that concomitantly drives direct tumor killing and antitumor immunity,” the scientists commented. “Arming naturally neoantigen-rich tumor cells with bifunctional therapeutics represents a promising cell-based immunotherapy for solid tumors and establishes a road map toward clinical translation.”
The post Cancer Cell-Based Vaccine Kills Tumors and Induces Long-Term Immunity appeared first on GEN – Genetic Engineering and Biotechnology News.
therapeutics
cell therapy
immunotherapy
vaccines
crispr
gene editing
medical

Wittiest stocks:: Avalo Therapeutics Inc (NASDAQ:AVTX 0.00%), Nokia Corp ADR (NYSE:NOK 0.90%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Spellbinding stocks: LumiraDx Limited (NASDAQ:LMDX 4.62%), Transocean Ltd (NYSE:RIG -2.67%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Asian Fund for Cancer Research announces Degron Therapeutics as the 2023 BRACE Award Venture Competition Winner
The Asian Fund for Cancer Research (AFCR) is pleased to announce that Degron Therapeutics was selected as the winner of the 2023 BRACE Award Venture Competition….














