Life Sciences
Engineered Cells Become Drug Factories with Avian Assistance
A group of researchers from Rice University reports the creation of autonomous cells with the ability to biosynthesize and genetically encode the amino…
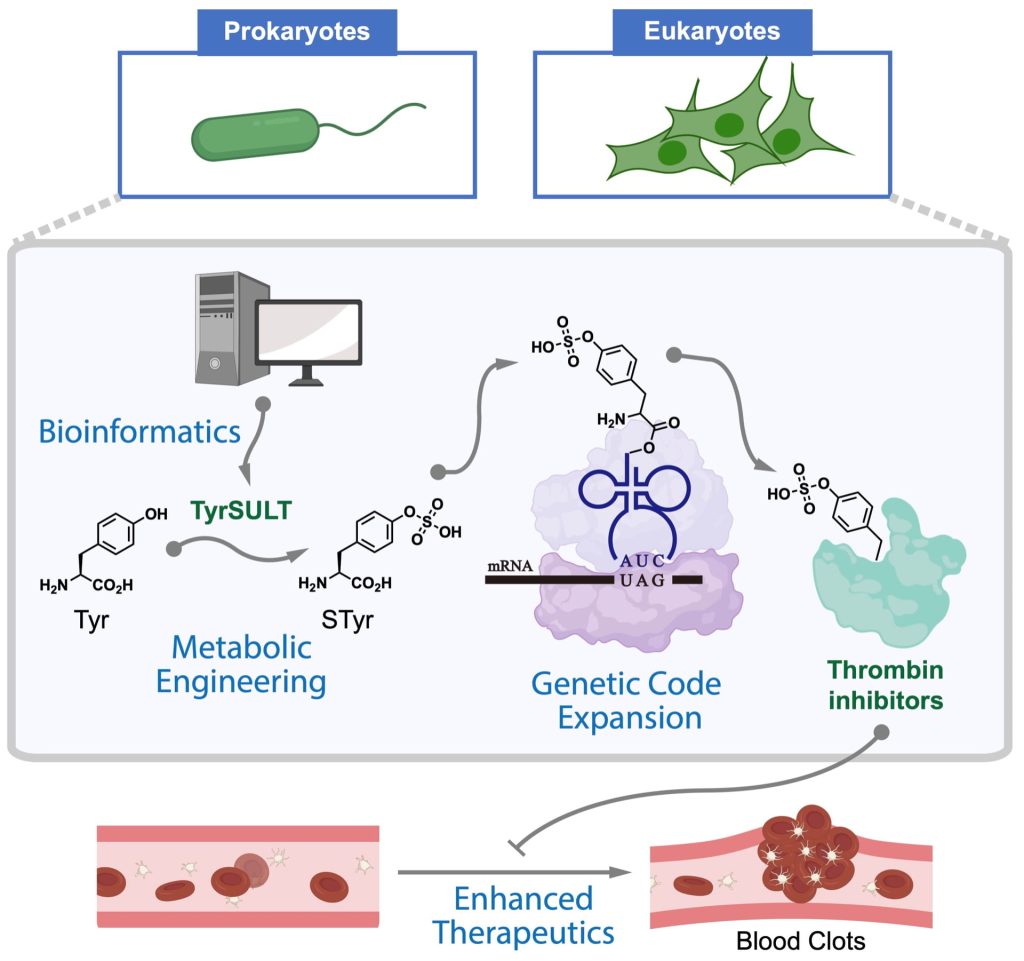
The 20 canonical amino acids (in addition to a few others like pyrrolysine and selenocysteine) are used for the biosynthesis of proteins. However, genetic code expansion technology, which enables the site-specific incorporation of noncanonical amino acids (ncAAs) into proteins in living cells, has transformed research. In addition, it has the potential to lead to the development of new therapeutics.
Now, a group of researchers from Rice University reports the creation of autonomous cells, both prokaryotic and eukaryotic, with the ability to biosynthesize and genetically encode the amino acid sulfotyrosine (sTyr)—an important protein post-translational modification with low membrane permeability and a key building block to program living cells that express therapeutic proteins.
One key component to the researchers’ success was the discovery that the bird, the crested ibis, provided the clues needed to unlock the production of sTyr.
Not only did the proof-of-concept study produce mammalian cells that synthesize sTyr for the first time, but the researchers also made cells that enhanced the potency of thrombin inhibitors—anticoagulants used to prevent blood clotting.
The study appears in the paper, “Unleashing the potential of noncanonical amino acid biosynthesis to create cells with precision tyrosine sulfation,” published in Nature Communications.

“In nature, most of our species are made with 20 canonical building blocks,” said Han Xiao, PhD, assistant professor of chemistry, biosciences, and bioengineering at Rice University. “If you want to add an additional building block, you need to think about how to make it. We solved that problem: We can ask the cell to make it.”
“But then we have to have the translational machinery to recognize it. And a special codon to encode this new building block,” Xiao continued. “With this study, we’ve fulfilled all three of these requirements.”
In the past, scientists would feed chemically synthesized noncanonical amino acids into cells. Having the cell do the work is far more efficient, Xiao said, but it required the discovery of a new transferase enzyme with tyrosine pockets that could bind sulfate. That lock-and-key combination could then be used as the foundation for a variety of catalysts.
And, the researchers have the crested ibis to thank for helping make that leap. When the lab of Peter Wolynes, PhD, professor of biosciences, materials science & nanoengineering, and physics & astronomy at Rice University, compared genome databases, they found sulfotransferase 1C1 in the crested ibis.

“We got lucky,” Xiao said. “Ibis is the only species doing this, which was discovered by a sequence similarity search of genomic information. After that, we asked if they can figure out why this enzyme recognizes tyrosine but our human sulfotransferase cannot.”
The genetic encoding of ncAAs with distinct chemical, biological, and physical properties requires the engineering of bioorthogonal translational machinery, consisting of an evolved aminoacyl-tRNA synthetase/tRNA pair and a “blank” codon. To achieve this, the researchers mimicked the ibis’ ability to synthesize sTyr and incorporate it into proteins.
The Xiao lab employed a mutant amber stop codon to encode the desired sulfotransferase, resulting in a completely autonomous mammalian cell line capable of biosynthesizing sTyr and incorporating it with great precision into proteins.
These engineered cells, the authors wrote, can produce “site-specifically sulfated proteins at a higher yield than cells fed exogenously with the highest level of sTyr reported in the literature.” They used the cells to prepare highly potent thrombin inhibitors with site-specific sulfation.
“Now, through this new strategy to modify proteins, we can totally change a protein’s structure and its function,” Xiao said. “For our thrombin inhibitors models, we showed that putting an unnatural building block in the drug can make the drug much more potent.”
The researchers expect to use the combination of bioinformatics and computationally enhanced screening to produce a library of biosynthesized noncanonical amino acids.
The post Engineered Cells Become Drug Factories with Avian Assistance appeared first on GEN – Genetic Engineering and Biotechnology News.

Wittiest stocks:: Avalo Therapeutics Inc (NASDAQ:AVTX 0.00%), Nokia Corp ADR (NYSE:NOK 0.90%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Spellbinding stocks: LumiraDx Limited (NASDAQ:LMDX 4.62%), Transocean Ltd (NYSE:RIG -2.67%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Asian Fund for Cancer Research announces Degron Therapeutics as the 2023 BRACE Award Venture Competition Winner
The Asian Fund for Cancer Research (AFCR) is pleased to announce that Degron Therapeutics was selected as the winner of the 2023 BRACE Award Venture Competition….














