Government
Harnessing Cell Therapy’s Cancer-Killing and Tissue-Reviving Potential
Now that cell therapies—and genetically engineered cell therapies—are a mainstay of modern medicine, scientists are working to find more applications…
Scientists have long conceived of using living cells as treatments for human ailments. In the 19th century, various kinds of animal cells were injected into people in attempts to treat age-related diseases or cancer. Fortunately, the field took on a more serious face in the mid 20th century, when researchers’ understanding of the human immune system greatly improved.
In the late 1950s, the American physician Edward Donnall Thomas and other scientists began treating leukemia patients by irradiating their bodies to eradicate the cancer and giving them infusions of bone marrow cells from healthy donors to restore their blood-forming systems. The donors’ tissues rarely engrafted into the recipients, however, and many patients died from serious immune reactions or infections.
Thomas found more success when he sourced the bone marrow cells from an identical twin of the recipient, which led to a remission of the leukemia until it eventually recurred. The key, he and others learned, was genetic similarity of cell surface receptors called human leukocyte antigens (HLAs) to prevent recipients’ immune systems from rejecting the foreign donor tissue. Over the years, he and others developed ways of HLA-matching donors with suitable recipients, as well as immunosuppressive treatments that helped prevent the transplanted cells attacking the recipients’ tissues.1,2 Eventually, bone marrow transplantation became an important therapy for certain blood cancers and certain other diseases.
These developments also sparked another line of research. Thomas and others noticed that donor immune cells would sometimes help eradicate rogue tumor cells that remained in recipients’ bodies. Researchers surmised that infusing immune cells alone into patients’ bodies could help combat blood cancers—a vision that came to fruition years later when advances in genetic engineering facilitated the development of chimeric antigen receptor (CAR) T-cell therapies. These involve extracting a patients’ T cells, genetically engineering their receptors to target specific cancer cells, and infusing them back into patients’ bloodstreams. In what the U.S. Food and Drug Administration (FDA) called a “historic action,” the first CAR T-cell therapy, tisagenlecleucel, was approved in 2017 to treat certain patients with acute lymphoblastic leukemia for whom standard therapy had been unsuccessful.3 Since then, a number of CAR T-cell therapies have been approved for a range of blood cancers.
Now that cell therapies—and genetically engineered cell therapies—are a mainstay of modern medicine, scientists are working to find more applications for them. Many are tinkering with immune cells to make them work for broader groups of patients and to direct them against solid tumors, which has so far proven challenging with CAR T cells. Others, meanwhile, are tinkering with stem cells to revive damaged tissues in degenerative diseases, in the hope of tackling progressive neurodegenerative diseases such as multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS). GEN spoke with a number of scientists and company leaders to learn more about the challenges and successes at these two therapeutic frontiers.
Making CAR T cells better killers
The California-based company ImmPACT Bio is working on developing more effective CAR T-cell therapies. One limitation of some popular CAR T-cell therapies is that cancer cells often lose the B-cell receptor they’re designed to target—the CD19 protein, for instance—which can allow the malignancy to recur, says Sumant Ramachandra, MD, PhD, Imm-PACT Bio’s president and CEO. He explains, “There’s usually an antigen loss by malignant B cells when they’re under selection pressure by CD19-CAR therapy.”
ImmPACT Bio specializes in developing logic-gated CAR T-cell therapies. In these therapies, the CAR T cells are engineered to follow logical rules, such as targeting either one of two antigens. The company’s lead product is a CAR T-cell construct designed to target either the CD19 or CD20 B-cell receptor.
The construct was developed by one of the company’s scientific founders—Yvonne Chen, PhD, of the University of California, Los Angeles (UCLA). UCLA researchers recently concluded a safety study of 10 patients with relapsed or refractory non-Hodgkin lymphoma who received an infusion of their own T cells after the T cells had been engineered to express the construct. In the Phase I data reported in a preprint, nine patients responded to the therapy and seven achieved complete remission after two months.4
Importantly, no patients experienced neurotoxicity or severe levels of cytokine release syndrome—common side effects of conventional CAR T-cell therapies and a reason why the therapies are given in the inpatient setting. Fewer side effects mean that outpatient administration is possible. “If your therapy is very tolerable,” Ramachandra elaborates, “you can now increase access.”
ImmPACT Bio researchers are now planning another Phase I study to test the CAR T-cell therapy with the company’s own industrialized manufacturing process, alongside efforts to develop other logic-gated CAR T-cell therapies for solid tumors.
Reengineering T cells for solid tumors
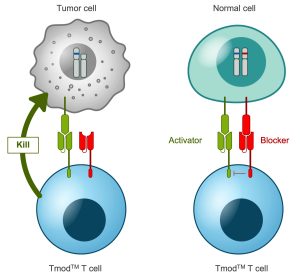
A2 Biotherapeutics, a firm based in California, is also pursuing a CAR T-cell therapy for solid tumors. One of the challenges involved is that many of the CAR T-cell therapies tested so far target tumor-associated antigens that are also expressed in healthy tissue, which the T cells end up attacking. “With regular cell therapies … there is this on-target, off-tumor toxicity that can happen,” explains Talar Tokatlian, PhD, a senior scientist at A2 Biotherapeutics. “Depending on the tissue, it can be very problematic.”

The company’s solution consists of logic-gated CAR T-cell constructs that target tumor-associated antigens but are directed not to target cells with antigens that are expressed only on healthy cells. One of the company’s leading candidates targets the carcinoembryonic antigen (CEA) present in colorectal and certain other cancers, but is blocked by HLA-A*02, a commonly expressed antigen in healthy tissues. Tokatlian says that for a subset of patients whose tumors have lost expression of HLA-A*02, the treatment would selectively eliminate tumor cells while protecting healthy tissues.
One 2022 study reported that these CEA-targeting “Tmod” cells selectively killed CEA-expressing tumor cells and avoided HLA-A*02-expressing cells in mouse cancer models.5 The team observed similar results with Tmod cells that target the tumor-associated antigen mesothelin and are blocked by HLA-A*02.6
The limitation, Tokatlian points out, is that these therapies apply only to patients who are heterozygous for HLA-A*02 and have complete loss of the antigen in tumor cells. The goal is to find more combinations of activating and blocking antigens to identify other subgroups that could benefit. “Once we show that this works,” she says, “then the next step is to make sure we can apply this therapy to a lot of different patients.”
Mobilizing natural killer cells
Boston-based Catamaran Bio is using another immune cell type entirely to tackle solid tumors. Natural killer (NK) cells, part of the innate immune system, have several advantages over T cells in that they’re capable of killing tumor cells even if they don’t express the target antigen. In addition, the way that NK cells identify foreign cells makes them less likely to attack a recipient’s tissues if they’re derived from a different person—facilitating the development of off-the-shelf therapies that don’t need to be manufactured individually for each patient. And NK-cell therapies are expected to be much safer than the CAR T-cell therapies, suggests Alvin Shih, MD, CEO of Catamaran Bio.
The challenge with developing any cellular therapy for solid tumors, however, is that the tumor microenvironment is inherently immunosuppressive, often harboring cytokines like transforming growth factor-b (TGFb) that have inhibitory effects on immune cells. That’s why Catamaran Bio researchers have engineered NK cells, derived from peripheral blood, to carry a TGFb dominant negative receptor that enables the cells to capture the cytokines without activating any intracellular pathways and shuttle them out of the tumor microenvironment. Rather than using a virus to introduce the receptor-encoding genes into the NK cells, the researchers use a transposon-based system. According to Shih, this system is more efficient and allows for the insertion of larger genes.
In preclinical studies, Catamaran Bio scientists engineered these TGFb-capturing NK cells to target the HER2 receptor present in breast tumors.7 Mice with HER2-expressing tumors that received the NK cells survived longer than controls. “There’s a significant difference,” Shih remarks, “between the placebo-treated and the CAR-NK-treated animals, both in terms of tumor volume as well as survival.”
Harnessing mesenchymal stem cells
Meanwhile, the New York–based Brainstorm Cell Therapeutics is deploying mesenchymal stem cells (MSCs), which play various supportive roles in the body, to stall tissue loss. “Some cells within our bodies … have these amazing properties in and of themselves,” says Stacy Lindborg, PhD, executive vice president and chief development officer at Brainstorm.
Lindborg says Brainstorm’s therapy, NurOwn, has a multipotent mechanism of action that can tackle progressive diseases including the autoimmune disease MS as well as ALS, which leads to paralysis and death. The treatment consists of MSC cells that are isolated from individual patients’ bone marrow, induced to express neurotrophic factors, and delivered into patients’ cerebrospinal fluid, from where the MSC cells migrate to areas of inflammation. In some animal models of neurodegenerative diseases, implanting MSC cells that secrete neurotrophic factors had a neuroprotective effect.8,9
Brainstorm recently published results for a Phase II study of 18 patients with nonrelapsing, progressive MS who received multiple treatments of NurOwn.10 The therapy appeared to be safe, and 19% of patients saw improvements in a walk speed test after 28 weeks. Interestingly, researchers also observed an increase of neuroprotective proteins and a decrease in inflammatory biomarkers in treatment recipients’ cerebrospinal fluid—an observation also made in a Phase III study of NurOwn in 263 ALS patients.
However, that study didn’t meet its primary endpoint, with 33% of NurOwn recipients seeing an at least 1.25-point change in disease progression on an ALS functional rating scale after 28 weeks, compared with 28% of placebo-receiving patients—a difference the FDA didn’t consider sufficient to grant NurOwn a Biologics License Application.11 Buoyed by revised results, Brainstorm is now resubmitting its request. (The company found that the original results had been distorted by a statistical error in the analyses.12 Revised results for the study show that NurOwn significantly slowed disease progression on a secondary endpoint for a patient subgroup with less severe disease.)
Repairing motor neurons in ALS
Clive Svendsen, PhD, executive director of the Board of Governors Regenerative Medicine Institute at Cedars-Sinai Medical Center in Los Angeles, and his colleagues are also using stem cells to tackle neurodegenerative diseases, including retinitis pigmentosa, Parkinson’s disease, and ALS.13
Their approach, however, is based on a neural progenitor cell line derived from a human fetal cortical tissue sample isolated many years ago. The cells are genetically engineered to express glial cell line–derived neurotrophic factor (GDNF), which promotes neuron survival. The strategy is to transplant the cells directly into the spinal cord; they then migrate to sites of degeneration and differentiate into GDNF-secreting astrocytes, a cell type that supports motor neurons.
Svendsen and his colleagues recently tested these cells in a Phase I/IIa study, transplanting them into one side of the lumbar spinal cord of 18 ALS patients, who received immunosuppressive drugs to prevent transplant rejection. The treatment appeared safe.14 Notably, tissue analysis of 13 individuals who had died from ALS showed that the implanted cells survived and secreted GDNF. “That’s remarkable given we didn’t even know if they’d survive in an adult spinal cord, let alone in the environment of ALS,” Svendsen says.
The team is planning another study that involves implanting the stem cells into ALS patients’ motor cortexes to tackle the degeneration at the upper motor neurons. Svendsen notes that slowing disease progression requires treating both sites. He acknowledges that the road ahead is long. “This is a marathon,” he remarks. “It’s a series of safety steps, leading to a therapy which could protect both sides of the system.”
References
1. Storb R. Edward Donnall Thomas (1920–2012). Nature 2012; 491: 334.
2. Blume KG, Weissman IL. E. Donnall Thomas (1920–2012). Proc. Natl. Acad. Sci. U.S.A. Published 2012; 109(51): 20777–20778.
3. Rose S. First-Ever CAR T-cell Therapy Approved in U.S. Cancer Discov. 2017;7(10): OF1.
4. Larson SM, Walthers CM, Ji B, et al. CD19/CD20 Bispecific Chimeric Antigen Receptor (CAR) in Naïve/Memory T Cells for the Treatment of Relapsed or Refractory Non-Hodgkin Lymphoma. medRxiv. Published September 14, 2022. Accessed: October 13, 2022. DOI: 10.1101/2022.09.13.22279873.
5. Sandberg ML, Wang X, Martin AD, et al. A carcinoembryonic antigen-specific cell therapy selectively targets tumor cells with HLA loss of heterozygosity in vitro and in vivo. Sci. Transl. Med. 2022; 2(14): eabm0306. DOI: 10.1126/scitranslmed.abm0306.
6. Tokatlian T, Asuelime GE, Mock J, et al. Mesothelin-specific CAR-T cell therapy that incorporates an HLA-gated safety mechanism selectively kills tumor cells. J. Immunother. Cancer 2022; 10(1): e003826. DOI: 10.1136/jitc-2021-003826.
7. Richardson C, Moore F, Alvarez A, et al. Allogeneic natural killer cells engineered to express HER2 CAR, interleukin 15, and TGF-beta dominant negative receptor effectively control HER2+ tumors. Abstract presented at: American Association for Cancer Research Annual Meeting; April 8–13; New Orleans, LA. Cancer Res. 2022; 82(12_Suppl.): Abstract 555. DOI: 10.1158/1538-7445.AM2022-555.
8. Sadan O, Shemesh N, Barzilay R, et al. Mesenchymal stem cells induced to secrete neurotrophic factors attenuate quinolinic acid toxicity: A potential therapy for Huntington’s disease. Exp. Neurol. 2012; 234(2): 417-427. DOI: 10.1016/j.expneurol.2011.12.045.
9. Dadon-Nachum, Ben-Zur T, Srugo I, et al. Therapeutic Effect of Myogenic Cells Modified to Express Neurotrophic Factors in a Rat Model of Sciatic Nerve Injury. J. Stem Cells Regen. Med. 2012; 8(1): 21–27. DOI: 10.46582/jsrm.0801004.
10. Cohen JA, Lublin FD, Lock C, et al. Evaluation of neurotrophic factor secreting mesenchymal stem cells in progressive multiple sclerosis. Mult. Scler. J. Published online September 14, 2022. DOI: 10.1177/13524585221122156.
11. U.S. Food and Drug Administration. Update on Amyotrophic Lateral Sclerosis (ALS) Product Development. Published March 2, 2021. Accessed September 26, 2022.
12. Cudkowicz ME, Lindborg SR, Goyal NA, et al. A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve 2022; 65(3) :291–302. DOI: 10.1002/mus.27472.
13. Behrstock S, Ebert A, McHugh J, et al. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene The. 2006; 13(5): 379-88. DOI: 10.1038/sj.gt.3302679.
14. Baloh RH, Johnson JP, Avalos P, et al. Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: a phase 1/2a trial. Nat. Med. 2022; 28: 1813–1822. DOI: 10.1038/s41591-022-01956-3.
The post Harnessing Cell Therapy’s Cancer-Killing and Tissue-Reviving Potential appeared first on GEN – Genetic Engineering and Biotechnology News.
manufacturing
therapeutics
cell therapy
stem cells
biologics
biomarkers
medical
application
therapy
fda
research

Here Are the Champions! Our Top Performing Stories in 2023
It has been quite a year – not just for the psychedelic industry, but also for humanity as a whole. Volatile might not be the most elegant word for it,…
AI can already diagnose depression better than a doctor and tell you which treatment is best
Artificial intelligence (AI) shows great promise in revolutionizing the diagnosis and treatment of depression, offering more accurate diagnoses and predicting…
Scientists use organoid model to identify potential new pancreatic cancer treatment
A drug screening system that models cancers using lab-grown tissues called organoids has helped uncover a promising target for future pancreatic cancer…













