Life Sciences
In Biomanufacturing, Integration and Intensification Go Hand in Hand
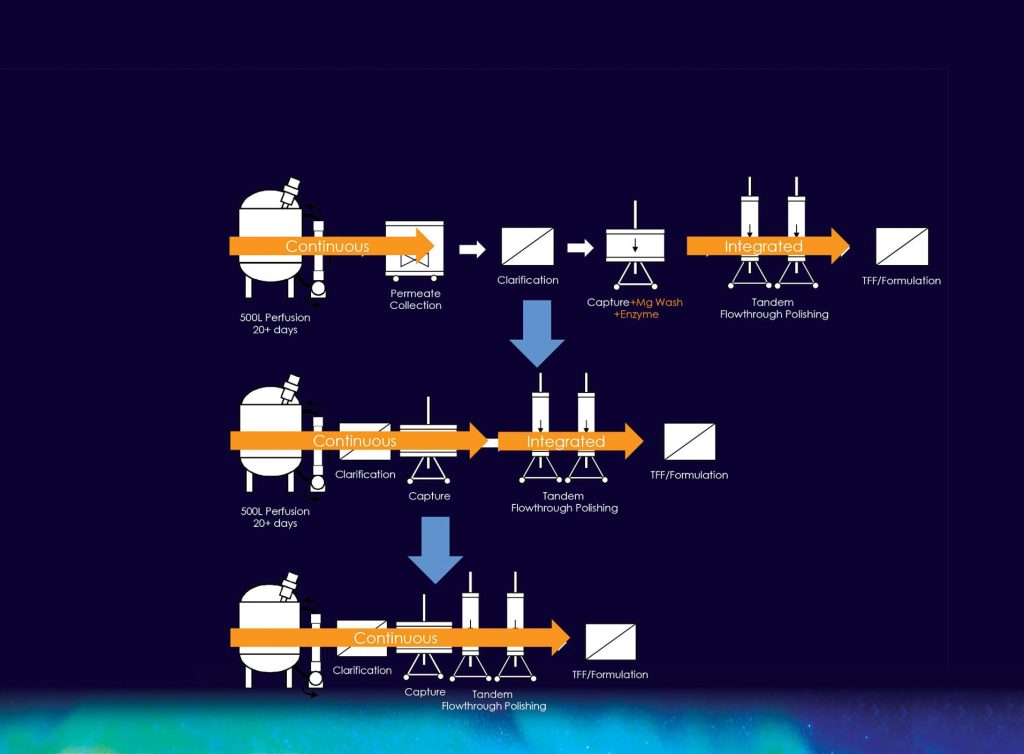
Continuous manufacturing prioritizes integration and intensification. Integration is about harmonizing upstream and downstream operations, and intensification…
The term “sustainability” usually brings to mind consumer products—not life-saving biologics such as recombinant proteins, monoclonal antibodies, and vaccines. Somehow, with biologics, we overlook the resources that are consumed and the wastes that are generated. But these can be considerable, especially when biologics are produced in sprawling biomanufacturing facilities that rely on conventional fed-batch processes. Fortunately, there is a more sustainable alternative: continuous manufacturing.
In general, continuous manufacturing prioritizes integration and intensification. Integration is about harmonizing upstream and downstream operations, and intensification is about shrinking facilities, consuming fewer resources, and producing fewer wastes.
Despite offering these advantages, continuous manufacturing has been limited to ambitious, forward-thinking companies that have been willing to try new technologies—and to adjust operations accordingly. For example, there are continuous manufacturing technologies that allow the first column operation to capture a protein molecule in near real time. This linkage can result in enhanced process economics, but it requires that upstream and downstream development teams work closely together.
Reducing footprints, lowering costs
“A much smaller perfusion bioreactor can match the productivity of a deep-tank fed-batch stainless-steel one,” says Mark Brower, PhD, a director at Merck & Co. Perfusion technology enables a shift from large intermediate pooling vessels to smaller surge tanks. In general, it supports continuous flow between unit operations.
“Continuous integrated manufacturing and single-use systems allow us to shrink our facility footprint,” Brower notes. “But you have to look at how your process steps are connected and understand how a continuous approach can introduce new variables and expand the design space.”
Merck & Co. has developed a completely continuous stream that starts with a perfusion bioreactor and flows into and through surge vessels that connect unit operations. An automation system links all steps including chromatography, viral inactivation, depth filtration, polishing, viral filtration, and ultrafiltration. In addition to an ultrafiltration step that occurs online, the product can go through an additional ultrafiltration step offline to further concentrate the product and prepare it for final formulation into drug substance.
Full automation allows cohesion and coordination between unit operations, enabling a “lights out” approach—a 24/7 operation requiring only a single shift. “We have demonstrated our integrated continuous manufacturing through ultrafiltration product in a GMP environment for clinical application,” Brower asserts. “We can extend the principles to other proteins that use similar technologies with a good probability of success.”
Additional technological advances are needed to facilitate intensified processing. Some unit operations, such as flow-through chromatography, can be handled much the way they are in conventional batch processes. But some unit operations, such as ultrafiltration, are more challenging.
Another difficulty is that most perfusion membranes typically transmit less than 100% of the protein into the permeate stream, and transmission can decrease over time, making downstream processes harder to control. Next-generation perfusion membranes are needed that can provide a more consistent stream profile and support larger bioreactors with higher flux.
“We are also still looking for robustness enhancements in the more standard tools like single-use pH and flow sensors,” Brower adds. “Plus, a common question from regulators concerns contamination. New tools are needed in rapid microbe detection to accurately detect confirmed microbial contamination before reaching an actual limit.”
Automation is crucial but remains a barrier to entry. No standards exist, and often companies develop specialized code. Industry consortia such as the National Institute for Innovation in Manufacturing Biopharmaceuticals and the BioPhorum Operations Group are investigating plug-and-play automation solutions to alleviate this challenge.
Streamlining exosome production
To facilitate the design, engineering, and manufacture of novel exosome therapeutic candidates, Codiak BioSciences introduced the EngEx platform. During the platform’s development, several technical challenges had to be overcome, recalls Aaron Noyes, EngD, Codiak’s vice president of integrated drug substance development. For example, it was necessary to identify single-use bioreactor films that would be compatible with high-performance cell culture media. (Certain films proved incompatible with Codiak’s HEK293 producer cells.)

Another challenge was the need to identify chromatography resins for exosome purification. Because exosomes occur in polydisperse, heterogeneous populations, resins that are selective enough to remove impurities from exosomes can be identified only through extensive, iterative screening.
Early on, it was critical to establish a benchmark for exosomes that would qualify as “Codiak exosomes.” This task entailed the development of protocols to quantify and characterize exosomes and to implement quality control.
The use of an endonuclease to digest DNA prior to chromatography capture is common in gene therapy manufacturing and an approach Codiak employed initially. But enzymatic digestion early in the process was not conducive to continuous processing. So, instead of relying on enzymatic digestion, Codiak developed a strategy that incorporates chromatography optimization and post-capture digestion. The company also developed a tandem chromatography step. Doing so entailed the alignment of buffer compatibility, complementary selectivity, capacities, flow rates, and residence times.
“Bioburden-reduction filtration was similarly a challenge,” Noyes details. “We evolved from 0.8 µm to sterilizing-grade filtration. To filter a 0.2 µm diameter particle through a 0.2 µm diameter filter pore demands a refined operation.”
Migrating from fed-batch to perfusion operations was technically demanding. In perfusion, cell retention filters with 0.2 to 0.65 µm pores are typically employed to permit the passage of antibodies that are approximately 0.01 µm in diameter. In the purification of 200 nm exosomes, a similarly proportioned approach would require filters with pore sizes of over 10 µm, which are not readily available. Even if they were, they would permit the passage of cells.
Extensive optimization is required to allow perfusion bioreactors to operate at steady state for several weeks, and improved technology is required to enable continuous clarification. “Relatively few gamma-irradiated depth filters are commercially available, and this space is ripe for disruption by alternative technology,” Noyes observes. “For large bionanoparticles like exosomes, filtration throughput can be low, owing to the high number of particles in the permeate yielded by the permissive cell retention filters used for perfusion. Since exosomes bind diatomaceous earth, capacities on depth filters can be low, requiring large installations, high costs, and frequent change-out.”
Another welcome development would be a proliferation of systems capable of simultaneously controlling multiple operations. Such systems would streamline and simplify process integration and automation. In addition, assay improvements are needed. “Breakthroughs in rapid titer assays would turbocharge the implementation of process analytical technology,” Noyes declares. “They would enable increasingly sophisticated control of interrupts, diversion, and process drift.”
Simplifying protein manufacturing
Sunflower Therapeutics, a public benefit company, plans to make biologics manufacturing more accessible by creating systems that are deployable anywhere in the world. “You should not have to be a bioprocess expert to manufacture proteins,” says Laura Crowell, PhD, Sunflower’s director of research and development. “We are developing simple processes, hardware, and software to produce recombinant proteins for applications such as therapeutics, vaccines, and alternative food products.”

A simple process needs a simple host. “We believe microbes can offer many benefits compared to conventional CHO cells,” Crowell elaborates. “For example, the yeast Pichia pastoris grows really fast, is easy to engineer to produce the desired protein, and has fewer impurities that impact purification.”
Exploiting the simplicity afforded by Pichia, the company has developed small-scale, automated systems that enable end-to-end protein manufacturing. Users can insert cells, set recipes, walk away, and return a few days later to retrieve purified bulk protein from a collection refrigerator.
Sunflower has developed a perfusion bioreactor called the Daisy Petal. This bioreactor, which is available to early access customers, has been shown to work in either of two closed, end-to-end systems—the benchtop Daisy system or the Dahlia system, which can produce about 10 kg of protein annually while occupying a space smaller than 50 m2.
Sunflower recently demonstrated that the same bioprocess could be run on either the Daisy or Dahlia prototypes to make high-quality protein. The prototypes were tested in unconventional settings, such as carpeted offices and bare warehouses, to demonstrate the potential of the closed systems.
Sunflower’s purification approach relies on straight-through chromatography, reducing the need for buffer conditioning between chromatography steps. Buffer requirements, processing times, and manufacturing footprints are reduced because there are no hold tanks to necessitate salt and pH level adjustments.
“While a connected system is more efficient,” Crowell notes, “it does also put more constraints on the conditions selected during process development.” The full multicolumn chromatography process is approached and optimized as a single unit operation to enable simple and rapid deployment.
“The hardest part in transitioning from a batch operation to a continuous, automated one is that you do not necessarily get to see what is going on between steps,” Crowell adds. “Our software is designed to monitor and control the important parameters. Operators do not need to be involved in every decision.
“In addition, during process development, you really have to be aware of how each choice, like the temperature of the bioreactor, can affect the rest of the connected process. The lines become blurred between upstream and downstream.”
Modeling processes to improve decision making
To help biopharmaceutical firms develop efficient processes and reduce manufacturing costs, Biopharm Services developed BioSolve, a set of bioprocess analysis tools. For example, the BioSolve Process 9 add-on module can be used to prepare an updated version of Biopharm’s Process Mass Intensity (PMI) report as well as a new Environmental report. The PMI report shows how much water, raw material, and consumables are used to produce 1 kg of product. The Energy report calculates operating energy load and operating carbon footprint.
Andrew Sinclair, the founder and president of Biopharm Services, notes that manufacturing operations consume less energy than clean room operations, which require that humidity and temperature be kept within specified bounds. “To get at energy usage, specifically in clean room operations, the challenge from a modeling perspective was that [we had to do more than] look at the service and support equipment,” he says. “We also had to estimate the volume of the facility.”
Volume estimates allowed the assessment of the total energy and productive facility use for the first time. The operation efficiency metric, Process Facility Intensity, estimates g/year of output per unit volume of process clean room space.
To assess the impact of process intensification on manufacturing costs, Biopharm Services “looked at cost of goods, capital intensity, and sustainability,” Sinclair remarks. Also, the company considered several single-use bioreactor setups (up to 5,000 L for fed-batch processes and 2,000 L for perfusion processes) and three intensifying options.
Cost of goods was not impacted by intensification. However, there were significant benefits. For example, capital intensity was reduced 15–20%. (The more intense the process, the larger the reduction in capital requirements.)
PMI, a ratio of the mass of inputs to the mass of outputs, served as the basis of material efficiency calculations. The overall PMI values reflected the large volumes of water used, particularly in operations intensified by perfusion-based processes. PMI values specifically for buffers and media salts were about 40 for batch processes and about 70 for perfusion processes.
To date, changes in the amounts of plastic used have not been evaluated for processes that have been intensified by a shift to single-use consumables. A typical 2,000 L batch process uses about 200 g of plastic/g of product. Efficiency increases with intensification. For the equivalent capacity, the intensive process uses 100 g of plastic/g of product. In models built by BioPharm Services, greater intensification equated to better use of clean room space. Indeed, a 40–50% productivity improvement was calculated.
Unlike batch processing technologies, many new intensification technologies still need to be fully optimized in terms of operating costs, capital costs, and outputs. “If we focus on monoclonal antibodies,” Sinclair says, “the area that needs most to be optimized is solution preparation and management, which easily accounts for 30–40% of total costs.”
The post In Biomanufacturing, Integration and Intensification Go Hand in Hand appeared first on GEN – Genetic Engineering and Biotechnology News.
manufacturing
therapeutics
antibodies
gene therapy
vaccines
biologics
biopharmaceutical
chromatography
application
therapy

Wittiest stocks:: Avalo Therapeutics Inc (NASDAQ:AVTX 0.00%), Nokia Corp ADR (NYSE:NOK 0.90%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Spellbinding stocks: LumiraDx Limited (NASDAQ:LMDX 4.62%), Transocean Ltd (NYSE:RIG -2.67%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Asian Fund for Cancer Research announces Degron Therapeutics as the 2023 BRACE Award Venture Competition Winner
The Asian Fund for Cancer Research (AFCR) is pleased to announce that Degron Therapeutics was selected as the winner of the 2023 BRACE Award Venture Competition….














