Life Sciences
Only 1 of 25 cancer drug developers fairly included minority patients over five-year window, BMJ analysis finds
New clinical trial diversity plans, which FDA will soon explain further, were featured in the government spending package that President Joe Biden signed…
New clinical trial diversity plans, which FDA will soon explain further, were featured in the government spending package that President Joe Biden signed late last week and are coming at a time when they’re very much needed, according to a new analysis published in the BMJ today.
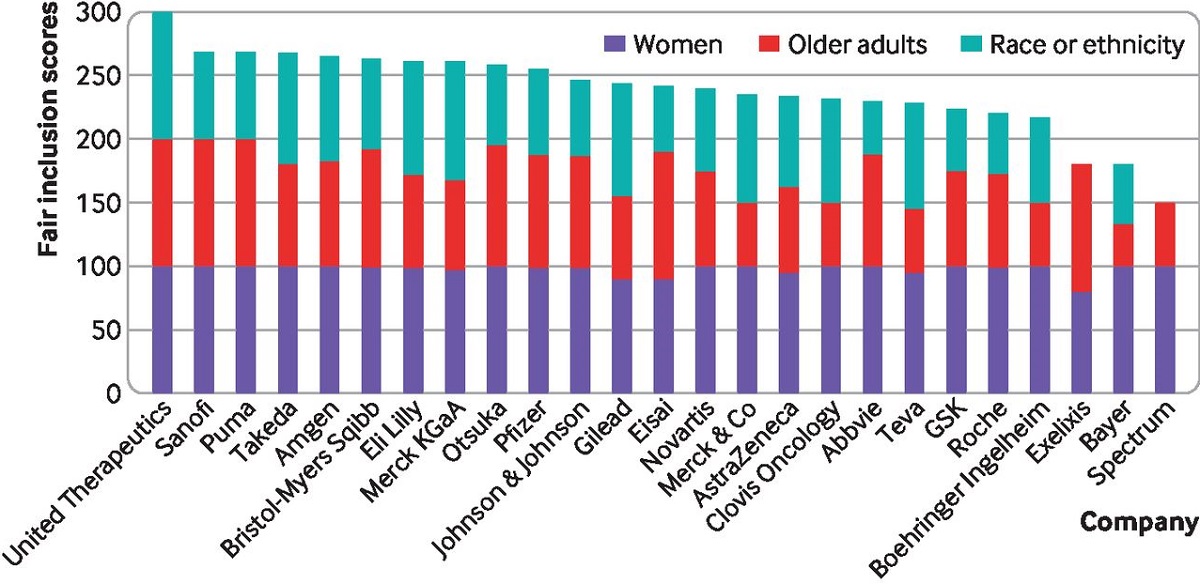
Of the 25 companies reviewed for the new diversity rankings, United Therapeutics was the only one with a perfect fair inclusion score of 100, but seven others — Puma Biotechnology, Sanofi, Takeda, Amgen, Bristol Myers Squibb, Eli Lilly, and Merck KGaA — received a score in the top quartile on clinical trial diversity performance, according to the analysis from Stanford, Yale and Bioethics International researchers, which was supported by a grant from the Susan G. Komen Foundation.
More than half of the 25 sponsors participating (56%) fairly included women, the researchers said, but just five sponsors (20%) fairly included older adults and only one sponsor (4%) fairly included racially and ethnically minoritized patients in trials. For individual products’ pivotal trials, the analysis found that 80% fairly included women, 24% fairly included older adults, and 5% fairly included racially and ethnically minoritized patients.

At the product level, 42% (25/59) of the cancer therapeutics reviewed included transparent data on the proportion of racial and ethnic minority participants in their trials, the research showed.
The so-called fair inclusion score, which is informed by data from 64 pivotal clinical trials of novel drugs and biologics approved between 2012 and 2017, considers transparency of reporting and the representation of three demographic groups that are often underrepresented in clinical trials in comparison to the disease-specific population: sex (percentage of participants that were female), age (percentage of adults older than 64) and race/ethnicity (percentage of participants identifying as Black, Asian and/or Latinx).
 Jennifer Miller
Jennifer Miller“The lack of diversity in clinical research is a social justice and a public health problem,” co-author Jennifer Miller, founder of Bioethics International and an associate professor at Yale School of Medicine, said in a statement.
While only a few sponsors have done well in the rankings, the authors noted that “most have substantial room for improvement on their inclusion of older adults and racially and ethnically minoritized patients, and to a lesser extent women.”
The rankings follow a Government Accountability Office report from last month that found that after more than three decades of instituting government policies to improve clinical trial diversity, certain racial and ethnic groups, as well as adolescents, older adults, women, low-income individuals, and individuals from rural communities “remain consistently underrepresented in cancer clinical trials.”
Moving forward, sponsors will soon be required to submit to FDA a “diversity action plan” for Phase III trials, including specifying enrollment goals upfront.
But the FDA has the ability to waive such a requirement if an indication is too small or for other discretionary reasons. Congress is mandating that FDA publish guidance on such action plans with more details, including on whether to grant a sponsor’s request to waive the requirement.
The agency will have two years to issue a report on what the diversity action plans have done up until then, in terms of boosting the enrollment of minorities and typically underrepresented populations.
therapeutics
biologics
fda
research
clinical trials
clinical research

Wittiest stocks:: Avalo Therapeutics Inc (NASDAQ:AVTX 0.00%), Nokia Corp ADR (NYSE:NOK 0.90%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Spellbinding stocks: LumiraDx Limited (NASDAQ:LMDX 4.62%), Transocean Ltd (NYSE:RIG -2.67%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Asian Fund for Cancer Research announces Degron Therapeutics as the 2023 BRACE Award Venture Competition Winner
The Asian Fund for Cancer Research (AFCR) is pleased to announce that Degron Therapeutics was selected as the winner of the 2023 BRACE Award Venture Competition….














