Life Sciences
Prescient unveils latest big hitter CellPryme-A at prestigious Boston CAR-TCR meeting
Special Report: Prescient has unveiled the latest technology in its cancer-fighting portfolio CellPryme-A at the 7th annual CAR-TCR Summit in … Read…
Prescient has unveiled the latest technology in its cancer-fighting portfolio CellPryme-A at the 7th annual CAR-TCR Summit in Boston, the world’s pre-eminent forum in the CAR and TCR fields of cellular immunotherapy.
Clinical stage oncology company Prescient Therapeutics (ASX:PTX) has unveiled a novel adjuvant/neoadjuvant named CellPryme-A for enhancing cellular immunotherapies.
CellPryme-A is designed to be administered to cancer patients as an intravenous infusion in combination with cellular immunotherapy, such as CAR-T cell therapy, to address the hostile tumour microenvironment that cellular immunotherapies face, thereby enhancing those therapies.
Neoadjuvant therapies are delivered before the main treatment, to reduce the size of a tumour or kill cancer cells that have spread. Adjuvant therapies are delivered after primary treatment, to destroy remaining cancer cells.
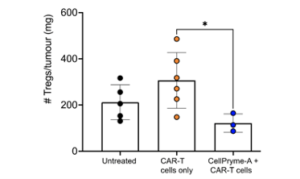
In animal models CellPryme-A reduces the number of suppressive regulatory T cells surrounding solid tumours that counteract the effectiveness of CAR-T and other cancer therapies.
CellPryme-A demonstrated superior tumour killing and survival in pre-clinical studies. And notably its effects were even greater when used together with Prescient’s CAR-T manufacturing technology, CellPryme-M.
CellPryme-A is now ready for clinical testing and can be incorporated into clinical studies of existing cell therapies. This opens up another avenue for collaboration with external parties and potential commercialisation for Prescient.
CellPryme-A has been developed by Prescient in collaboration with the world-renowned Peter MacCallum Cancer Centre in Melbourne, with Prescient owning the resultant intellectual property, which is the subject of a patent application.
Key CellPryme-A benefits
- Enhances tumour killing by conventional CAR-T cells, especially strong benefits when used in conjunction with CellPryme-M
- Improved host survival
- Reduces problematic Treg cells by 66%
- Increases ability of T cells to penetrate solid tumours
- Cytotoxic T cells by 400%
- Helper T cells by 300%
- Dramatically increases ability of CAR-T cells to expand within the host
- Doubles CAR-T cell expansion
- When used in conjunction with CellPryme-M:
- Cytotoxic T cells increased by 900%
- Helper T cells increased by 600%
Combating problematic Treg cells
The objective of CellPryme-A administration is to counteract the hostile “cold” tumour microenvironment that is known to dampen the tumour killing ability of CAR-T cells and similar types of cellular therapy.
This is achieved by reducing the numbers of suppressive regulatory T cells (Tregs) that infiltrate into the tumour.
Tregs are some of the most immunosuppressive immune cells in the body. In autoimmune diseases, it is the loss or dysfunction of Tregs that results in autoimmunity – where the immune system becomes hyper-activated in the absence of Tregs.
The opposite is true in cancer, where tumours can evade immune surveillance by recruiting Tregs to the tumour.
Tregs can create an immunosuppressive environment that prohibits efficient tumour killing, through the release of immunosuppressive cytokines and alterations in metabolic demand.
CellPryme-A reduces the numbers of problematic Tregs in solid tumours using an immune-competent mouse model of HER2+ colon cancer (MC38 colon carcinoma) and a conventional HER2-targeting CAR-T cell therapy.
Twice-weekly administration of CellPryme-A reduced the numbers of Tregs per mg of tumour by two-thirds, after one week of treatment.

Working to tackle solid tumours
CAR-T therapies have shown promising results in blood cancers. However, PTX’s senior vice president of Scientific Affairs Dr Rebecca Lim said solid tumours continue to pose challenges to CAR-T therapies.
“It is crucial that we address the immunosuppressive contributions of regulatory T cells as they significantly inhibit the efficacy of even the best-in-class CAR-T cells,” she said.
“The synergies between CellPryme-A and our CellPryme-M platform gives cellular immunotherapy the best chance at success based on these animal studies.”
PTX managing director and CEO Steven Yatomi-Clarke said CellPryme-A is a distinct but complementary addition to CellPryme-M, expanding its stable of cell therapy enhancements.
“Together with Prescient’s next-generation CAR platform, OmniCAR, Prescient has placed itself enviably at the forefront of cellular immunotherapy by creating technologies that overcome the challenges facing the field.”
“These challenges – which include targeting an array of antigens; post infusion control; cell exhaustion and a suppressive tumour microenvironment – simply must be overcome to bring this promising new class of therapies to more patients, and to conquer different malignancies.
“We now have a comprehensive suite of technologies to address all these challenges.”
The unveiling of CellPryme-A follows a series of positive announcements from Prescient, including a collaboration with the US’s largest cancer centre University of Texas MD Anderson Cancer Center.
PTX and MD Anderson are aiming to create best-in-class, adaptable CAR-T cell therapies to treat haematological malignancies.
PTX has always been a proponent of licensing from and collaborating with other world-leading cancer research institutions including Yale, Oxford, UPenn and Peter MacCallum Cancer Centre.
Yatomi-Clarke said CellPryme-A is the latest in a portfolio of platform cell technologies that can not only create innovative programs for Prescient but are also enabling technologies that open third party commercialisation opportunities.
“They can be utilised separately, or together for synergistic benefit, depending on the needs of a particular cell therapy program,” he said.
Join a CellPryme-A briefing
Prescient will be holding a live and interactive briefing on Tuesday, 27th September at 12pm (AEST), to discuss CellPryme-A in more detail. Click here to register.
This article was developed in collaboration with Prescient Therapeutics, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
The post Prescient unveils latest big hitter CellPryme-A at prestigious Boston CAR-TCR meeting appeared first on Stockhead.
manufacturing
therapeutics
cell therapy
immunotherapy
application
therapy

Wittiest stocks:: Avalo Therapeutics Inc (NASDAQ:AVTX 0.00%), Nokia Corp ADR (NYSE:NOK 0.90%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Spellbinding stocks: LumiraDx Limited (NASDAQ:LMDX 4.62%), Transocean Ltd (NYSE:RIG -2.67%)
There are two main reasons why moving averages are useful in forex trading: moving averages help traders define trend recognize changes in trend. Now well…
Asian Fund for Cancer Research announces Degron Therapeutics as the 2023 BRACE Award Venture Competition Winner
The Asian Fund for Cancer Research (AFCR) is pleased to announce that Degron Therapeutics was selected as the winner of the 2023 BRACE Award Venture Competition….














