Medtech
FDA: Glucose and Insulin Devices Can Now Work Together
The FDA says an iCGMs can link to an AIDs! The U.S. Food and Drug Administration (FDA) has cleared Abbott’s FreeStyle Libre 2 and FreeStyle Libre 3 integrated…
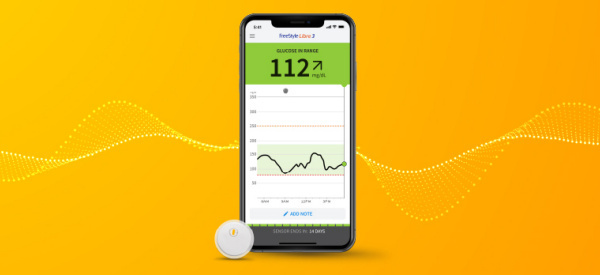

The FDA says an iCGMs can link to an AIDs! The U.S. Food and Drug Administration (FDA) has cleared Abbott’s FreeStyle Libre 2 and FreeStyle Libre 3 integrated continuous glucose monitoring (iCGM) system sensors for integration with automated insulin delivery (AID) systems. This clearance is a significant step forward in diabetes management technology, and this integration could affect how millions of people around the world administer their insulin. Abbott says its FreeStyle Libre portfolio has “changed the lives of 4.5 million people across more than 60 countries.”
The FreeStyle Libre 2 and FreeStyle Libre 3 sensors provide real-time glucose readings, making it easier for people with diabetes to make informed decisions about their treatment. With the integrated system, people with diabetes can adjust their insulin doses based on their glucose readings without having to manually enter data into their devices. The new integrated system should help reduce the risk of hypoglycemia, or low blood sugar, which can be a life-threatening complication of diabetes.
The FDA’s clearance includes use for two notable groups: children as young as 2 years old with Type 1 diabetes and pregnant women. Both groups need effective diabetes management. According to the Centers for Disease Control and Prevention (CDC), “diagnosed cases of Type 1 and Type 2 diabetes are surging among youth in the United States.” The CDC found that between 2001 and 2017, the number of people under the age of 20 with Type 1 diabetes increased by 45%. Regarding pregnant women, the CDC says diabetes during pregnancy increases the risk of preterm births, stillbirths, and birth defects.
So when will we be able to get iCGMs that link to AIDs? Abbott says it’s working with insulin pump manufacturers, including Tandem and Insulet, and foresees that integrated systems will be available in the U.S. later this year. And, hopefully, at a reasonable price. Jared Watkin, Abbott’s Senior Vice President, Diabetes Care, says, “The FreeStyle Libre portfolio is already the most prescribed CGM in the United States, and, with the integration of automated insulin delivery systems, people in the U.S. will soon have an affordable option to pair with insulin pumps.”

ETF Talk: AI is ‘Big Generator’
Second nature comes alive Even if you close your eyes We exist through this strange device — Yes, “Big Generator” Artificial intelligence (AI) has…
Apple gets an appeals court win for its Apple Watch
Apple has at least a couple more weeks before it has to worry about another sales ban.
Federal court blocks ban on Apple Watches after Apple appeal
A federal appeals court has temporarily blocked a sweeping import ban on Apple’s latest smartwatches while the patent dispute winds its way through…














