Medtech
Nova Eye Medical eyes strong growth as it awaits FDA approval for iTrack™ Advance
Special Report: Nova Eye Medical is awaiting US FDA approval for its updated surgical device to treat glaucoma which is … Read More
The post Nova Eye…
Nova Eye Medical is awaiting US FDA clearance for its updated surgical device to treat glaucoma, which is forecast to significantly expand its market in the US.
Nova Eye Medical (ASX:EYE) is tackling two of the biggest diseases leading to blindness in the developed world – glaucoma and macular degeneration.
EYE managing director Tom Spurling said the company has developed surgical devices for treating patients with glaucoma and has a clinical-stage product for intermediate stage macular degeneration.
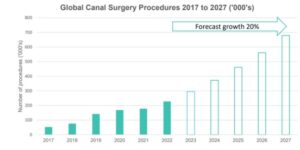
The market for surgical devices for the treatment of glaucoma is currently valued at US$700 million. Within this segment, EYE has pioneered the fastest growing part: canal surgery for glaucoma. Canal surgery is expected to grow at a CAGR of 20% between now and 2027.
The company holds more than 100 patents in relation to canal surgery, which is projected to grow to US $440 million per year over the next five years.
“Glaucoma is a progressive eye disease that can’t be cured and leads to irreversible vision loss unless there’s an intervention,” Spurling said.
“The idea is to slow it down..”
Spurling said glaucoma can be thought of as a failure of the eye’s drainage system, which can cause fluid to build up inside the eye, leading to excessive intraocular pressure and damage to the optic nerve.
iTrack clears blockages
EYE’s current iTrack product, a microcatheter indicated for canaloplasty, has been in the US market since 2008 and helps surgeons clear the blockages causing excessive fluid build-up in the eye.
iTrack Advance, a new generation product, is currently progressing through the US Food and Drug Administration (FDA)510(k) pathway.
“We anticipate FDA clearance for iTrack Advance any day now,” he said.
“It is a big catalyst for the company.”
High growth rate for canal surgery
Spurling said glaucoma surgical intervention is growing at 14.7% per annum, laser at 10% and medical treatment at 4.6%.
“Medications such as tropical drops are a very large market, but it is growing slowly due to poor compliance rates amongst patients,” he said.
“Canal surgery procedures are forecast to grow at 20% per annum over the next 5 years, faster than any other surgical device intervention option for glaucoma.”

EYE is among three major players in the segment along with Sight Sciences and New World Medical.
iTrack Advance to increase EYE’s market
Headquartered in Adelaide, EYE’s business is 70% in the US market, with the rest in Europe and China.
“We sell direct in the US, Germany and Australia, and through about 20 distributors in other key markets in China and Europe. We have our production in California and Dunedin on New Zealand’s South Island. EYE is one of the few companies in our space that owns all parts of the engineering and production process, a distinction that we are very proud of – and one that ultimately allows us to bring the most cutting-edge technologies to market,” Spurling said.
EYE’s 12-month revenue for its glaucoma surgical devices was A$15.2 million and is forecast to grow significantly following iTrack Advance’s US clearance, which will broaden the company’s US customer base by a factor of ~50.
Spurling said iTrack Advance improves surgical ergonomics without changing method of action, making the canaloplasty procedure more accessible and appealing to the much broader market of cataract and anterior segment surgeons.
“Currently our existing iTrack product is used by about 200 glaucoma surgeons in the US but there are about 1200 glaucoma specialists in total, and on top of that nearly 10,000 cataract and anterior segment surgeons,” he said.
“Cataract surgery is the world’s most common surgery and 20% of people who have cataracts have glaucoma.”
Spurling said with the improved ergonomics of iTrack Advance, EYE is now likely to appeal to cataracts surgeons doing a lot of cataract surgeries each week.
“Many cataract surgeons removing the clouded natural lens of their patients will, at the same time, treat their patient’s concomitant glaucoma.”
“This is the growth theme we are looking at,” he said.
Well-funded after capital raise
EYE completed an $8 million capital raise on March 2 through a two-tranche share placement to fund expansion of its glaucoma surgical device in the US, and in China and Europe.
Spurling said the placement which included the issue of 44.4 million shares was strongly supported by existing and new institutional investors.
The first tranche included 21,004,542 securities being offered to raise $3.78 million.
EYE issued the remaining 23,439,902 under the second trance to raise $4.22 million.
“We’ve raised enough money for the commercial launch of iTrack Advance and are scaling our commercial infrastructure in the US,” Spurling said.
This article was developed in collaboration with Nova Eye Medical, a Stockhead advertiser at the time of publishing.
This article does not constitute financial product advice. You should consider obtaining independent advice before making any financial decisions.
The post Nova Eye Medical eyes strong growth as it awaits FDA approval for iTrack™ Advance appeared first on Stockhead.

ETF Talk: AI is ‘Big Generator’
Second nature comes alive Even if you close your eyes We exist through this strange device — Yes, “Big Generator” Artificial intelligence (AI) has…
Apple gets an appeals court win for its Apple Watch
Apple has at least a couple more weeks before it has to worry about another sales ban.
Federal court blocks ban on Apple Watches after Apple appeal
A federal appeals court has temporarily blocked a sweeping import ban on Apple’s latest smartwatches while the patent dispute winds its way through…














