Cell division, i.e., the process through which a parent cell divides into two daughter cells, is fundamental to the growth, repair, and reproduction of living organisms. During cell division, chromosomes are pulled towards opposite spindle poles through the shortening of molecular ropes known as microtubules. Microtubules, which are composed of the protein tubulin, shorten with the help of “Pac-man”-like molecules that eat away at their tips. In most organisms, these Pac-man-like molecules are a group of proteins from the kinesin-13 subfamily that lead to microtubule shortening. Interestingly, during cell division in fission yeast, microtubule shortening also occurs in the absence of kinesin-13 proteins, suggesting the involvement of other proteins.
Recently, a team of researchers including Professor Masamitsu Sato and Ph.D student Yuichi Murase from the Department of Life Science and Medical Bioscience at Waseda University, Mika Toya from the Global Center of Science and Engineering at Waseda University, Junichiro Yajima from the Department of Life Sciences at the University of Tokyo, and Takahiro Hamada from the Department of Bioscience, Okayama University of Science conducted a study to identify these non-kinesin proteins.
It is known that proteins from the TOG/XMAP215 family such as Dis1 are involved in microtubule elongation in certain species. However, previous findings have indicated that even in cells without the Dis1 protein, the length of the microtubules is similar to that of normal cells. Elaborating further, Sato says, “Twenty years back, researchers considered TOG proteins as conventional microtubule stabilizers and believed that kinesin is the only factor responsible for microtubule shortening to carry chromosomes. But my observation through a microscope indicated that Dis1 acted like a de-stabilizer, making me curious about how exactly microtubule shortening occurs in yeast.”
In this study, which was published online on November 26, 2022, in Communications Biology, the team tested their hypothesis that Dis1 might be involved in microtubule shortening in yeast.
To arrive at their findings, the team conducted in vitro and in vivo experiments in yeast cells. They captured images of microtubules in yeast via fluorescence microscopy and examined how Dis1 modified microtubule length. Images captured within intervals of five seconds depicted that Dis1 promotes microtubule shortening through microtubule catastrophe—a process in which growing microtubules suddenly switch into a rapidly shortening state.
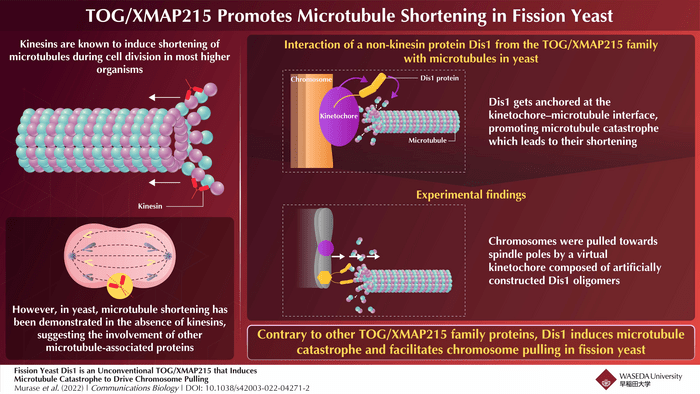
Before being split and pulled, chromosomes are held in place by microtubules emanating from the opposite poles. The team observed that Dis1 collects near the tip of the microtubule at the point in the chromosome where microtubules attach, known as the kinetochore. This suggests that Dis1 causes microtubule catastrophe by remaining anchored to the microtubule-kinetochore junction, thereby causing a sudden shortening of microtubules.
To test whether Dis1 is indeed involved in chromosome pulling, the team created artificial Dis1 oligomers which were then tethered to the chromosome arm region, to induce artificial chromosome pulling in yeast. The team observed smooth and frequent pulling of chromosomal arms by the microtubules connected to the artificial Dis1 kinetochore towards the spindle poles.
The interaction of Dis1 with the microtubules in fission yeast is unique and unexpected since TOG/XMAP215 proteins are known to be involved in microtubule polymerization, the process that leads to microtubule elongation in many organisms.
What are the long-term applications of these findings? Sato muses, “We envision that the molecular device created in this study can be a platform for the artificial chromosome segregation machinery, which might contribute to therapy for diseases that are caused by defects in chromosome segregation. Alternatively, it could be used for the construction of artificial cells that can segregate genetic materials to descendants independently.”
***
Reference
DOI: https://doi.org/10.1038/s42003-022-04271-2
Authors: Yuichi Murase1, Masahiko Yamagishi2, Naoyuki Okada1,3, Mika Toya1,4,5, Junichiro Yajima2,6,7, Takahiro Hamada8 and Masamitsu Sato1,5,9
Affiliations:
1Laboratory of Cytoskeletal Logistics, Department of Life Science and Medical Bioscience, Graduate School of Advanced Science and Engineering, Waseda University, 2-2 Wakamatsucho, Shinjuku-ku, Tokyo, 162-8480, Japan
2Department of Life Sciences, Graduate School of Arts and Sciences, The University of Tokyo, 3-8-1 Komaba, Meguro-ku, 153-8902, Tokyo, Japan
3Instituto de Biologia Molecular e Celular, Instituto de Investigacao Inovacao em Saude (i3S), Universidade do Porto, 208 Rua Alfredo Allen, 4200-135, Porto, Portugal
4Global Center for Science and Engineering, Faculty of Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku-ku, Tokyo 169-8555, Japan
5Institute for Advanced Research of Biosystem Dynamics, Waseda Research Institute for Science and Engineering, Graduate School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku-ku, Tokyo, 169-8555, Japan
6Komaba Institute for Science, The University of Tokyo, 3-8-1, Komaba, Meguro-ku, 153-8902, Tokyo, Japan
7Research Center for Complex Systems Biology, The University of Tokyo, 3-8-1, Komaba, Meguro-ku, 153-8902, Tokyo, Japan
8Department of Bioscience, Faculty of Life Science, Okayama University of Science, 1-1 Ridaicho, Kita-ku, Okayama-shi, 700-0005, Japan
9Institute for Medical-Oriented Structural Biology, Waseda University, 2-2 Wakamatsucho, Shinjuku-ku, Tokyo, 162-8480, Japan
About Professor Masamitsu Sato
Professor Masamitsu Sato is a distinguished researcher and professor at Waseda University’s Department of Life Science and Medical Bioscience with over 24 years of research experience. He received his doctoral degree from the University of Tokyo’s Department of Biophysics and Biochemistry. Professor Sato is a recipient of the JSPS Fellowship for Research Abroad and worked as a post-Doctoral fellow at Cancer Research UK’s London Research Institute from 2002 to 2006. He was awarded the Commendation for Science and Technology by Japan’s Minister of Education, Culture, Sports, Science and Technology in 2012. He has also received the Waseda University Research Award and the Waseda University Teaching Award. Professor Sato’s research currently focuses on the mechanisms of cell division and cell differentiation.
About Waseda University
Located in the heart of Tokyo, Waseda University is a leading private research university that has long been dedicated to academic excellence, innovative research, and civic engagement at both the local and global levels since 1882. The University has produced many changemakers in its history, including nine prime ministers and many leaders in business, science and technology, literature, sports, and film. Waseda has strong collaborations with overseas research institutions and is committed to advancing cutting-edge research and developing leaders who can contribute to the resolution of complex, global social issues. The University has set a target of achieving a zero-carbon campus by 2032, in line with the Sustainable Development Goals (SDGs) adopted by the United Nations in 2015.
To learn more about Waseda University, visit https://www.waseda.jp/top/en
Journal
Communications Biology
DOI
10.1038/s42003-022-04271-2
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Fission yeast Dis1 is an unconventional TOG/XMAP215 that induces microtubule catastrophe to drive chromosome pulling
Article Publication Date
26-Nov-2022