Medtech
Type 2 Diabetes Managed via Artificial Pancreas in Trial
An artificial pancreas could offer an alternative treatment for patients living with type 2 diabetes. The fully automated, closed-loop device, powered…
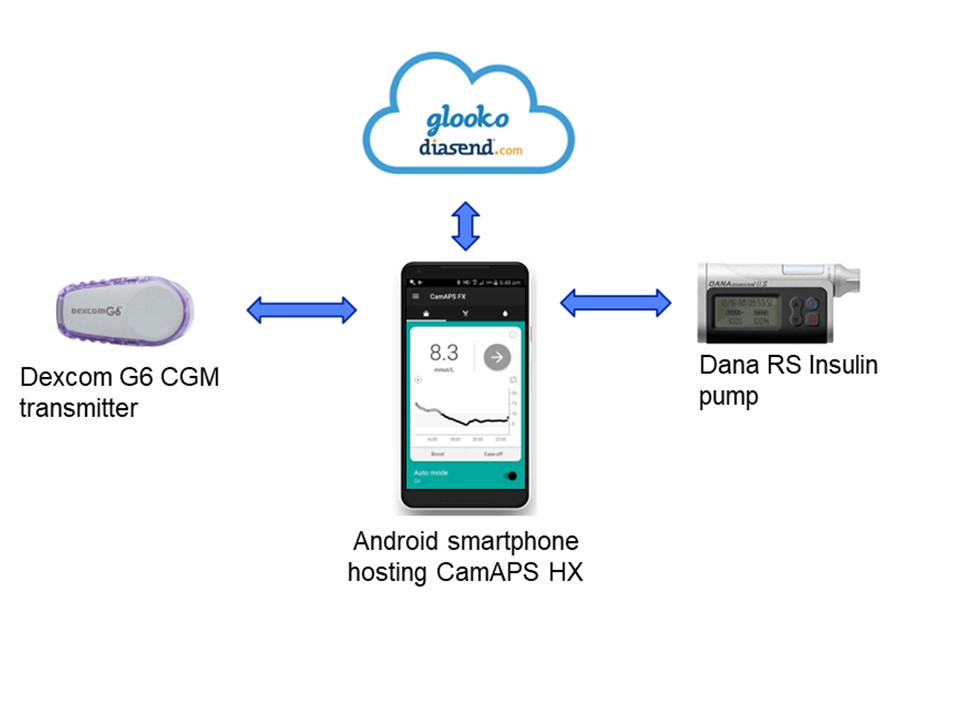
Scientists at the Wellcome-MRC Institute of Metabolic Science at the University of Cambridge have developed a fully automated closed-loop insulin delivery system that improved glucose control in adults with type 2 diabetes (T2D), without increasing bouts of low blood sugar levels (hypoglycemia) compared to standard insulin therapy. This convenient “artificial pancreas” that requires no input from users at mealtimes may offer a safe and effective way to improve the management of T2D.
Results from the open-label, single-center randomized crossover trial (clinicaltrials.gov registration# NCT04701424) were published in Nature Medicine (“Fully automated closed-loop insulin delivery in adults with type 2 diabetes: an open-label, single-center randomized crossover trial”).

Co-lead author of the study Charlotte Boughton, MBBS, PhD, from the Wellcome-MRC Institute of Metabolic Science at the University of Cambridge, said, “Many people with type 2 diabetes struggle to manage their blood sugar levels using the currently available treatments, such as insulin injections. The artificial pancreas can provide a safe and effective approach to help them, and the technology is simple to use and can be implemented safely at home.”
Earlier studies have successfully trialed hybrid closed-loop insulin delivery systems, but these required user inputs in the form of mealtime announcements that regulate boluses of insulin release. Such systems are currently available for patients with type 1 diabetes. The new insulin delivery system called CamAPS HX responds to the patient’s blood glucose levels. It includes a continuous glucose monitor, insulin pump, and an algorithm that predicts the amount of insulin required to maintain glucose levels within the target range, which modulates the delivery of insulin under the skin.
The current study tested the CamAPS HX system in 28 patients from the Wolfson Diabetes and Endocrine Clinic at Addenbrooke’s Hospital, which is part of Cambridge University Hospitals NHS Foundation Trust, and a local group of GP surgeries. The recruited patients were randomly allocated to two groups—the first group used the CamAPS HX system for eight weeks and then switched to the standard therapy of multiple daily insulin injections; the second group was on the standard therapy first and then switched to the CamAPS HX system after eight weeks.
The researchers assessed the proportion of time that patients spent with their glucose levels between 3.9 and 10.0 mmol/L. On average, patients using the CamAPS HX system spent two-thirds (66%) of their time within the target range, which was double that while on the standard therapy (32%). Next, the investigators measured the fraction of time patients spent with glucose levels above 10.0 mmol/L, and found patients on standard therapy spent two-thirds (67%) of their time at high glucose levels while patients using the CamAPS HX system spent half of that time (33%) at levels above 10.0 mmol/L. Moreover, the average glucose levels decreased from 12.6 mmol/L on the standard therapy to 9.2 mmol/L while using the CamAPS HX system, and glycated hemoglobin levels (HbA1c) decreased from 8.7% on standard therapy to 7.3% when using the CamAPS HX system.
Hypoglycaemia was not observed in patients in either group during the study. Co-lead author of the study, Aideen Daly, MBBS, also from the Wellcome-MRC Institute of Metabolic Science, said, “One of the barriers to widespread use of insulin therapy has been concern over the risk of severe ‘hypos’—dangerously low blood sugar levels. But we found that no patients on our trial experienced these, and patients spent very little time with blood sugar levels lower than the target levels.” The only serious adverse event during the trial was the hospitalization of one patient using the CamAPS HX system due to an abscess at the site of the pump cannula.
The investigators intend to conduct a larger multicenter study to confirm the safety and efficacy of the system and have submitted the device for regulatory approval.
The research was funded by the National Institute for Health and Care Research (NIHR) Cambridge Biomedical Research Centre.
The post Type 2 Diabetes Managed via Artificial Pancreas in Trial appeared first on GEN – Genetic Engineering and Biotechnology News.

ETF Talk: AI is ‘Big Generator’
Second nature comes alive Even if you close your eyes We exist through this strange device — Yes, “Big Generator” Artificial intelligence (AI) has…
Apple gets an appeals court win for its Apple Watch
Apple has at least a couple more weeks before it has to worry about another sales ban.
Federal court blocks ban on Apple Watches after Apple appeal
A federal appeals court has temporarily blocked a sweeping import ban on Apple’s latest smartwatches while the patent dispute winds its way through…














